With the collaboration of Gianfranco Huaman (OjoPublico, Peru), Rafael Neves (The Intercept, Brazil) and John Machado (El Mercurio, Ecuador)
Around 110 companies from 18 countries in the Americas, Asia and Europe produced, marketed and exported kits to detect Covid-19, including biotech giants such as Roche (Switzerland), Thermo Fisher (USA), BGI Group (China) and a large group of less-known Asian laboratories. Since February, the market for key supplies for manufacturing these tests is going through a scenario of high domestic demand in countries affected by the pandemic, and in those with no capacity to produce them in their own territory.
As in much of the world, Peru does not produce the reagents to manufacture such kits. “Reagents are bits of virus processed in high tech laboratories, which we do not have" said the president of the Public Health Committee of the Peruvian Medical Association, Augusto Tarazona. With these supplies, the two key tests that detect SARS-CoV-2 are possible: the molecular techniques that require a swab from the patient's nose or pharynx and its processing in a PCR machine, and immunoassays, which only need blood samples and that detect the body's response to Covid-19.
Both tests are used to detect various diseases, including HIV, is one of the most deadly diseases of the 20th century. To obtain the reagent kits, the Peruvian State usually purchases in the local market from companies operating as subsidiaries or authorized representatives of the group of pharmaceutical companies mentioned above. In other cases, reagents are received as a donation from entities such as the World Health Organization (WHO) or from countries where those tests are produced.
However, by March 6 -when Peru announced its Covid-19 patient zero- various countries of the world had already launched a global search to purchase and import the kits of molecular and immunoassay tests (also called rapid tests or serological tests). Weeks later, most of the producers of these tests -USA, Italy, Spain, France, Germany, United Kingdom, Iran and Brazil - were already suffering the worst onslaught of Covid-19 and had decided to reserve their products for domestic consumption.
For example, the U.S. Government authorized the domestic purchase of molecular tests to 19 companies in the country, among them the giants Thermo Fisher and Abbott Laboratories; while in Spain, the local Certest was one of the first companies contacted by the government to supply the kits. Meanwhile, another group of producers, such as China, Korea and Japan, are recovering from the initial outbreak of the disease occurred between January and March in Asia.
Since March 6, the Ministry of Health (Minsa) began to carry out tests among patients suspected thanks to the stock they had, from a donation from WHO and China. Since then, 19 thousand tests have been conducted with an average of 600 a day. In face of the shortage of reagents in Peru, the Executive announced in late March, the purchase of 350 thousand of molecular test kits, considered to be the most accurate for detecting viruses, and, complementarily, 1.4 million of rapid tests.

PURCHASES. Officials from the National Institute of Health (INS) review a shipment of rapid tests that arrived last week in Peru. The brand of these kits is Biomedomics and they were imported by a local company.
Photo: Minsa
At the time of going to press, OjoPublico established that Peru had bought 320 thousand molecular tests since the end of March from national companies that import their products from related pharmaceutical companies such as: BGI Group (China), TermoFisher, Cepheid and Abbot (US), and Roche (Switzerland). Meanwhile, the rapid kits purchased (still in unidentified quantities) come almost entirely from China, especially from Zhejiang Orient Gene Biotech, today one of the most favored companies by the high demand for these tests.
In this context, OjoPublico created a database of the largest global manufacturers of kits for detecting Covid-19 through molecular tests and immunoassay tests, based on information from the websites of producing countries, WHO, the Foundation for Innovative New Diagnostics from Switzerland and the websites of each of these companies. Below, an in-depth analysis of the market, its main actors and some of its representatives in Peru.
Roche of Switzerland: Global Pharmaceutical Power
The Roche group, dating back to the 19th century, is based in the city of Basel (Switzerland) and had global sales of more than US$61 billion in 2019. For this reason, Forbes magazine lists it as one of the 100 largest companies in the world. In the face of this pandemic, the pharmaceutical giant has a molecular test type called Cobas, designed to be used in the PCR machines developed by the same company.
Currently, Roche is authorized to sell their molecular kits and their Cobas 6800/8800 machine in some countries in Oceania, Asia and America. Additionally, their tests hold the CE marking, the certificate of quality required for sales in European Union countries. In a public statement released last March 24, the pharmaceutical company informed: “Roche can provide millions of tests a month.”
“[ROCHE TESTS] CAN ONLY BE PERFORMED IN THE COBAS 6800 SYSTEM," SAID OMAR MARTINEZ, THE COMPANY’S REPRESENTATIVE IN PERU.
In Lima, the Swiss multinational markets its products through Productos Roche QF SA, which started operations in 1961 and is currently managed by Roberto Taboada Gorbitz. This company was authorized to sell their molecular tests by the General Bureau of Medicines and Drugs (DIGEMID) on March 18, according to the company. Currently, Productos Roche QF is the leading importer of this type of tests for the domestic market: between 2012 and 2020 they had import operations up to US$50 million.
However, in Peru as in the USA, Brazil and Ecuador -the most affected countries by the pandemic in the region, and where Roche operates, the molecular tests are designed to be used in PCR machines developed by the Swiss pharmaceutical company. OjoPublico confirmed in documents from the U.S. Food and Drug Administration (FDA) and the National Health Surveillance Agency of Brazil that Roche reagents for detecting the virus "are for use in Cobas 6800/8800 automated systems".
The documents also specify that these molecular tests should only be performed by health personnel "who have received specific training in the use of [such] systems", in reference to the PCR equipment developed by Roche.
The communications manager for the Roche Group in Lima, Omar Martinez Reyes, also confirmed via email that the reagents of this company operate only on their machines, "in order to ensure a faster response" in the face of the pandemic. When asked if Roche reagents can work in PCR machines of other brands, Martinez said: “No, they may only be carried out in the Cobas 6800 system".
This same thing is happening in Ecuador. The medical director of Santa Inés Hospital in Cuenca, Luis Tamayo, told OjoPublico that "Roche Group conditions the delivery of their reagents to buying their machines." However, he said, his hospital has used reagents of this pharmaceutical company in equipment other than the Cobas 6800/8800 and have processed samples successfully. “Unfortunately, this is the way transnational companies work [...] their excuse was that their reagents do not work [with machines of other brands] to keep us as a captive market," said the doctor.
On March 29, El Mercurio newspaper from Ecuador reported the complaint Tamayo and other local hospitals filed against the laboratory. “Our reagents have to be run on our platforms," said Luis Villegas, Roche's representative in the neighboring country, when interviewed by said newspaper.
Consulted on this dependence between PCR machines and their kits, Roche Diagnostica Brasil, representative, Deborah Moreno, was explicit: the test can only be "exclusively analyzed in Cobas 6800/8800 systems". However, she left open the possibility that their kits “could be used in [other] equipment available in the market” only for clinical research.
The situation in Peru, Ecuador and Brazil has also been reported in Europe. “The risks of this practice have already become clear in the Netherlands, Roche in our case, was rationing the supply of certain testing materials [for Covid-19]," warned Irene Schipper of the Center for Research on Multinational Corporations (SOMO) in the Netherlands. “The effect was the Netherlands had to adjust its testing policy and run fewer tests than they might have wanted to serve the public health needs in this crisis in the best way" said Schipper.
On 26 March, the media Follow The Money from The Netherlands revealed that most laboratories in this country depend on machines and reagents produced by the Swiss biotechnological company. “The impact caused by the heavy reliance on Roche and its position in the market is quite negative. In fact, Roche has determined the testing policy in the Netherlands," Schipper indicated.
“ROCHE CONDITIONS THE DELIVERY OF THEIR REAGENTS TO BUYING THEIR MACHINES," SAID DR. LUIS TAMAYO FROM ECUADOR.
Despite the existing problem, the global media continue to report Roche offers to donate their reagent kits, without specifying that their PCR tests depend on their Cobas 6800/8800 machines.
In 2018,OjoPublico -as part of global research The Implant Files, led by the International Consortium of Investigative Journalists (ICIJ)- revealed that PCR machines, reagents and other equipment sent to Peru raised a total of 31 FDA alerts, between 2012 and 2014, for manufacturing defects.
On April 1, President Martin Vizcarra declared in a press conference that the Peruvian Government had purchased an unspecified number of molecular tests from Roche. However, the Minister of Health, Victor Zamora, indicated that same day that these tests had actually been donated by this company. We asked Minsa and Perú Compras for the detail of the operations and were told that ultimately more than 11 thousand Cobas kits for PCR had been purchased.
The first purchase order between the State and this company was dated March 24. Days later, between March 25 and 27, Productos Roche QF SA imported a shipment of supplies for US$18 thousand.
Thermo Fisher and Other US Pharmaceutical Companies
Thermo Fisher Scientific is a laboratory with headquarters in the USA and sales of US$24.3 billion in 2018, and is currently authorized to market their molecular Covid-19 tests in at least four countries: Canada, USA, Australia and Singapore. For example, in the last two jurisdictions, the sales permit was awarded to the subsidiary Life Technologies, a company also based in the USA, which was purchased by Thermo Fisher in 2014.
The Covid-19 kits from this laboratory are molecular, and have also been authorized for sale in countries in the European Union. “We are committed to the fight against this disease," said its president Marc Casper in a press release. Three years before, Human Rights Watch reported that the Government of China used Thermo Fisher equipment to monitor their citizens irregularly to create a DNA database.
In Lima, Thermo Fisher Scientific Peru SRL is in charge of importing products and supplies from the USA. Between 2012 and 2019, the company brought supplies for more than US$3 million. This company was founded by Manuel Barrios Arbulu and Julio Gallo Broel-Plater in 2012 and their first board of directors included the British citizen Anthony Hugh Smith, who today holds the same position in different subsidiaries of Thermo Fisher and is vice president of the headquarters in the USA.
Another local company importing Thermo Fisher and Life Technologies reagents is Belomed SRL, founded in 1988 in Lima and currently managed by Edgar Odría Guerra , a biotechnology equipment specialist. OjoPublico found out that this company sold an unspecified amount of molecular tests to the State at the end of last month. It should be mentioned that Belomed SRL has imported from Thermo Fisher around US$3 million in reagents for PCR machines between 2012 and this year.
We still ignore if Thermo Fisher Scientific Peru SRL or Belomed SRL are authorized to market their molecular tests in the country. We asked Digemid for information, to no avail. Our means also asked Thermo Fisher international press area, but they never replied.
Other major pharmaceutical companies in the USA, which produce, commercialize and export molecular tests, are: Abbott Laboratories, Laboratory Corporation of America (Labcorp) and Quest Diagnostics, placed next to La Roche in the Forbes ranking with annual sales of US$30 billion, US$11 billion and US$7 billion, respectively. This March 27, the US FDA authorized Abbott to commercialize their kits on American territory.
THERMO FISHER WAS ACCUSED OF FACILITATING IRREGULAR MONITORING IN CHINA, ACCORDING TO HUMAN RIGHTS WATCH.
This list also includes Cepheid of USA, which brings its molecular tests to Lima through the local Rochem Biocare del Peru S.A.C., the third most important of its kind in our market. Since 2012, this company has conducted import operations for US$8 million. Precisely, OjoPublico confirmed with Peru Shopping website that the State bought 50 thousand molecular tests from Rochem Biocare.
Another US laboratory, BioMedomics, producing rapid tests based on blood samples, also sent a shipment to Peru, which was sold by an unidentified national company to Minsa. Today BioMedomics has the CE marking, which gives it access to the European Union market.
Chinese Companies: Molecular and Rapid Tests
OjoPublico also confirmed that China has the largest number of companies registered with permits to commercialize Covid-19 PCR tests and rapid tests: at least 34 companies are authorized by regulatory entities in Europe, Oceania and Latin America to sell their kits. Some of the most visible for their molecular tests are: BGI Group (Beijing Genomics Institute), founded in 1999 in Shenzhen, Guangdong Province), and known for having participated in the Human Genome Project.
This company is one of the most important in its field in China and has been exporting its PCR tests to different countries in the world. For example, in Lima, the national company Peru, founded in 2013 and managed by Juan Alarcón Noboa, imported BGI reagents, and recently sold more than 109 thousand tests to the Peruvian State.
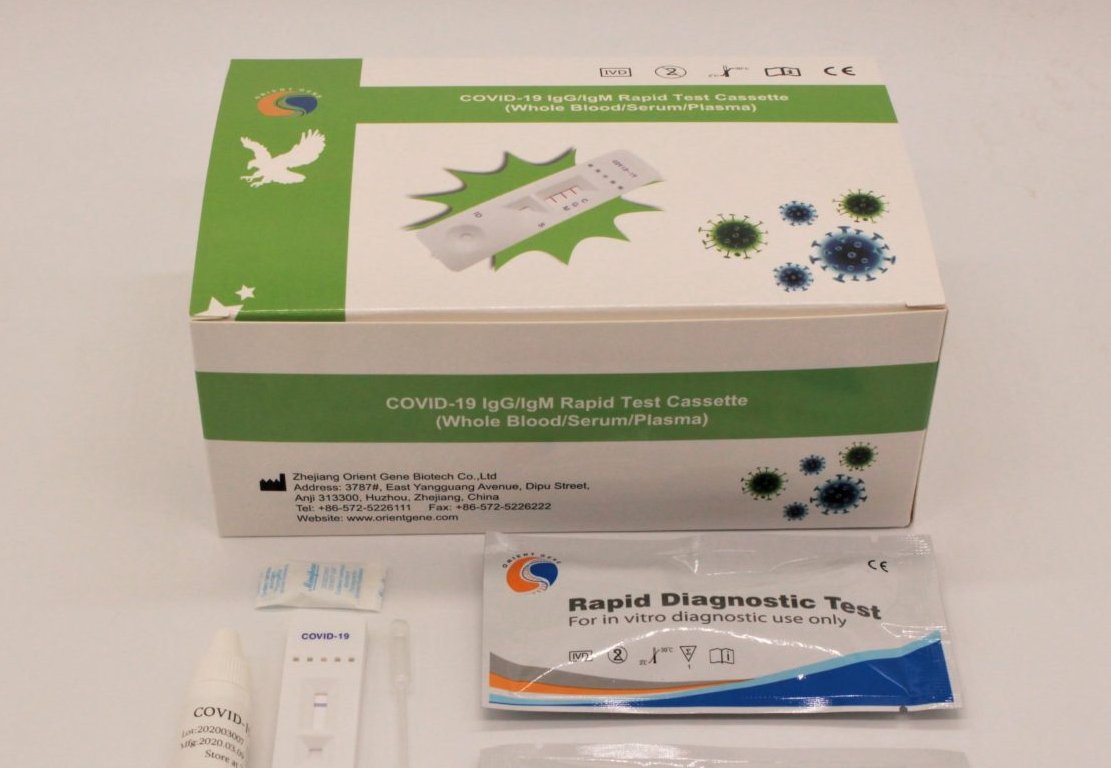
SUPPLIER. Zhejiang Orient Gene Biotech sells these rapid tests globally. A Peruvian company imported them and sold them to the State
Photo: Zhejiang Orient Gene Biotech
The list of molecular tests manufacturers in China also include: Easy Diagnosis and HealthCare Biotechnology, located in Wuhan, Hubei province), where the original outbreak of the pandemic occurred in December 2019. Meanwhile, some of the Chinese companies that produce rapid or serological tests include Zhejiang Orient Gene Biotech, founded in 2005 in Zhejiang province, and Wondfo Biotech, founded in 1992 in Guangzhou (Guangdong province).
Of the latter, Zhejiang Orient Gene Biotech is the one that attracts the most attention. In 2018, the Bill and Melinda Gates Foundation donated $1.1 million to help them to pre-qualify their products against malaria with WHO. In early January, shortly after China reported on the new coronavirus to the public, the national regulatory body gave the go-ahead for this company to be included in the Shanghai Stock Exchange board of innovation and science, known as STAR market.
In February, when Covid-19 expanded throughout Europe, Orient Gene Biotech made a major entrance in the stock market with arise in their stock prices and their market capitalization, a trend that has continued in recent weeks. Today the Chinese company is already selling their rapid tests in Australia, Peru and some countries in Europe.
Recently, Minsa purchased an unspecified amount of rapid tests from the local companies Multimedical Supplies y Nipro Medical Corporation, who in turn received them from Orient Gene Biotech and Core Technology, whose operations centers are also located in China.
Finally, the database built by OjoPublico includes 11 companies from South Korea and 11 from Singapore, whose tests to detect Covid-19 are commercialized within the framework of this outbreak. Of these, so far, only the South Korean Gene Finder and SD Biosensor have reached the Latin American market, as they are registered with the Argentinian Ministry of Health as authorized providers of molecular tests.
Peruvian Companies and their Foreign Suppliers
The State has also purchased supplies and materials for molecular tests (150 thousand units) from eight Peruvian companies that bring their products from abroad. The specific amounts were not provided by Perú Compras. The list of providers includes Merck Peruana, with a local presence since the 60's and is managed by Claudia Vertiz de la Flor. This company is related to Merck in Germany, a giant pharmaceutical company founded in the 17th century, with sales for U$17 billion last year.
“THE REAGENTS ARE [MANUFACTURED] IN HI-TECH LABORATORIES, WHICH WE DO NOT HAVE," SAID DR. AUGUSTO TARAZONA.
The other companies can be sorted according to their seniority in the market. The first group includes Inmunochem, Teccios, and Gen Lab del Peru, all founded between the 70's and 90's, and managed by Luis Quiroz Carpio, Walter Pramer Pérez and Jorge Ramirez Melgar, respectively. The second group is composed of Biodiz (founded in 2011), Biosix Import (2013), Laboratory Supply (2014) and Medical Insight (2019). The managers of these laboratories are Eduardo Peña Oganes, Jesús Ramírez Vera, Pedro Hidalgo Flores and Carlos Razzeto Ríos.
Inmunochen and Medical Insight have imported pharmaceutical products from Qiagen, which is based in Germany and authorized to sell their molecular tests in the US.
 Tienes reportajes guardados
Tienes reportajes guardados